Lithium is in high demand as the world shifts towards a cleaner and more sustainable future. To meet this demand, industry is developing processes to extract lithium from geologic fluids, like geothermal brines or produced brines from existing or abandoned oil and gas wells. The Salton Sea geothermal brines, located in Southern California, are estimated to hold around 15 million metric tons of lithium and can potentially be produced at 113,000 mt Li/yr. (1). Lithium concentration in Salton Sea is reported to be several hundred mg/l (2), which can be extracted effectively using direct lithium extraction (DLE) media, a recent technology advancement in which lithium manganese oxide or lithium titanium oxide act as a sorbent material. DLE media companies have told OLI that they are close to extracting lithium economically, from brines containing less than 50 ppm Li. So, the Salton Sea brine quality, with respect to the latest DLE media capabilities is relatively high.
Although the Salton Sea geothermal brine is relatively rich in lithium, extracting it has several challenges. Companies need to deal with the following chemistry challenges: the high salt concentration, precipitating metals, brine corrosivity, brine temperature, a sensitive environment and limited availability of fresh water. OLI has several client companies that are involved with producing Salton Sea lithium. They have described the impact of the above challenges on their efforts. OLI, being a company with expertise in Engineering Chemistry, works with these clients to overcome these challenges.
OLI’s crucial role in end-to-end lithium extraction design – optimizing process water
The key contribution by OLI was closing the chemistry and mass balance on an end-to-end lithium extraction design. Operators, by regulation, can use a limited amount of fresh water from nearby waterways. To make extraction viable, operators reuse and recycle their process water. This causes a buildup in dissolved and precipitating salts in the water circuit. A precise chemistry balance of the process is therefore essential, so that the plant can operate withing regulations once it is constructed. OLI’s Application Engineers used OLI Flowsheet: ESP, an electrolytes-based process simulator to complete this work. Direct lithium extraction (DLE) is the preferred extraction process and so the team created a private database containing the preferred media. This capability, plus the existing capability to simulate the multi-phase phase behavior of heavy brines made it possible to develop a comprehensive extraction design based on first principles science.
Technological expertise in Salton Sea lithium extraction
There were several reasons why OLI technology was ideal for these efforts based on technology capabilities. The Salton Sea brine contains Zn, Pb, Ni, Mn, Fe, Cu, Ca, Sr, Ba, As, F, B and other elements that display complex behaviors in water. Removing the metals before processing the brine is critical. Otherwise, the media column fouls, the chemical demand increases and the final product has high concentrations of contaminants. Zn, Pb, Ni, Mn, Fe, Cu can be removed by a combination of pH adjustment, carbonate addition and possibly oxidation. The other elements, Ca, Ba, As, F and B, are also removed by chemical treatment or adsorption, but given the chemical demand to remove them and their low market value, it is better to keep them from precipitating. Thus, there is a balance among removing metals that foul the process, minimizing chemical addition and recovering salable metals in a high purity form.
OLI solutions are well suited for this challenge because we developed thermodynamics to predict metals’ behavior years earlier for the oil and gas, chemical, corrosion, nuclear and water treatment industries. The value-added is providing a robust electrolyte tool to the design engineer so they can test ideas they sketched out on the white-board rapidly and accurately.
OLI has consulted on different aspects of Salton Sea brine including brine corrosivity, metals precipitation, lithium extraction, product purification and water reuse. The theme is consistent; how does an operator manage a hot, concentrated, corrosive brine while extracting valuable elements. The following plot shows the sensitivity of this brine to pH and temperature changes. The brine composition is pulled from a California Energy commission report (3) and contains 2300 mg/l Fe+2, 1200 mg/l Mg+2 and 660 mg/l Zn+2. Above 6.5 pH (calculated at 70 C), significant amounts of Fe(OH)2 precipitates. The liquid will remain relatively solids free below ~6.5 pH. This would be the ideal condition for extraction since it reduces the demand on pre-treatment filters or the potential for impurities to precipitate in the contactor.
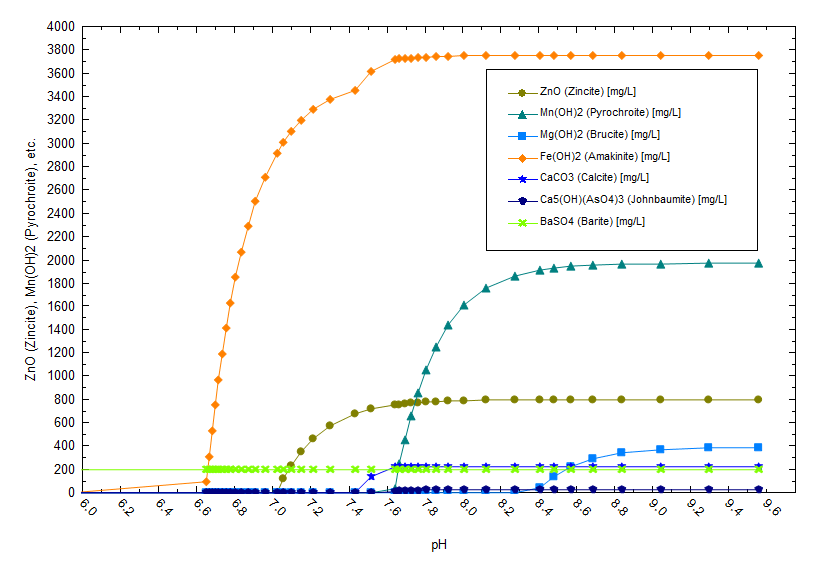
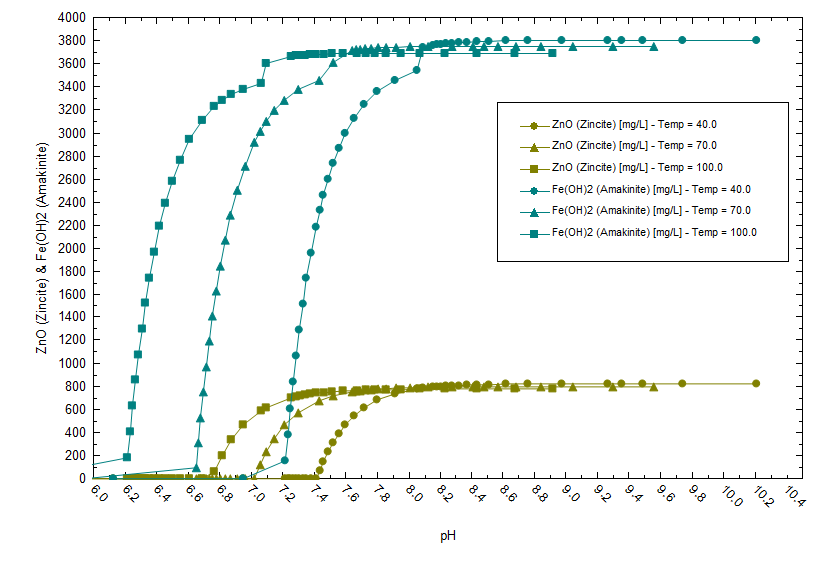
There is also a temperature effect on solubility as shown below. Fe(OH)2 precipitates at a lower pH when the temperature is higher (note the pH is computed at temperature).
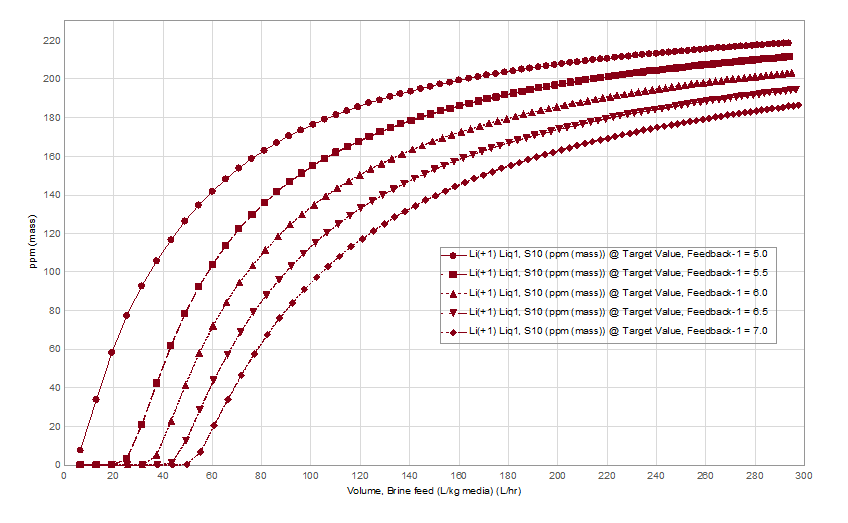
Therefore, the design engineers are challenged by three competing properties, feed brine temperature and the extent to which they cool it, the limit to which they can raise the brine pH and the need for a higher pH to optimize lithium extraction by the media. The pH effect on media performance is shown below. We used data from Wang et al, to set the pH-dependent lithium exchange coefficients on a model H2TiO3 media compound. The x-axis is the volume of pH-controlled, Salton Sea brine flowing across the media in ten-separation stages (column). At 5 pH, lithium breakthrough occurs after only a small volume of brine reacts with the media. By 7 pH, Li breakthrough occurs after 11x the brine passes across the media.
OLI Systems continues to pioneer innovative, scalable solutions for sustainable lithium extraction, addressing critical industry challenges with precision and expertise. If you’re ready to optimize your processes or learn how our tools can enhance your operations, contact us today and explore the difference OLI can make for your projects.
References
(3) Selective Recovery of Lithium from Geothermal Brines (ca.gov)

